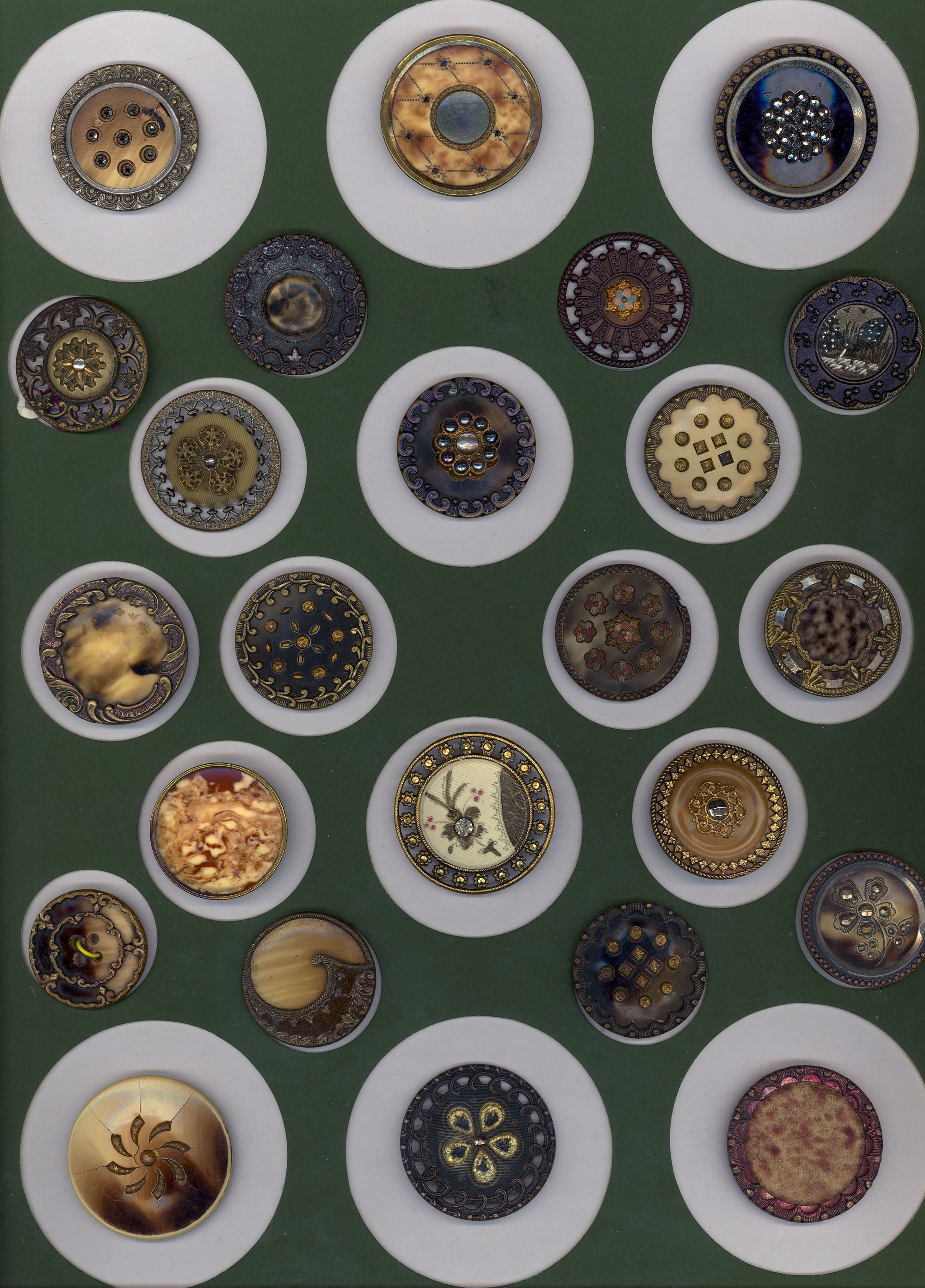
Compiled by Sue Dickout
Celluloid was invented in the middle of the 19th century, during the early days of chemical experimentation and synthesis. Many compounds were developed during this time which became important commercially, but many others were unprofitable or useless. What we know today as celluloid was a bit of both. Celluloid was developed from cellulose, the building block of wood fiber and cotton fiber. Cellulose treated with strong nitric acid formed nitrocellulose, which found some uses as an explosive (gun cotton). Efforts by English chemists in the 1860 to 1870 period to stabilize and manipulate it with solvents were moderately successful, but unprofitable. A few years later, an American inventor developed their materials by combining the natural tree product camphor with the nitrocellulose. The camphor dissolved the nitrocellulose, but also affected the structure of the product and performed as a plasticizer. This development was ultimately successful and became trademarked as Celluloid.
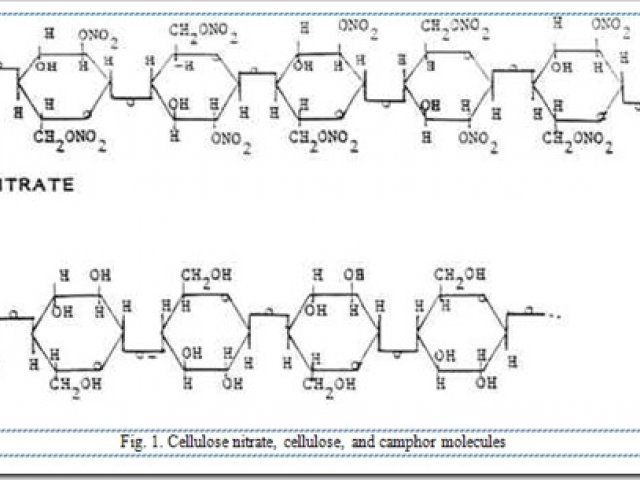
Fig. 1. Cellulose nitrate, cellulose, and camphor molecules
In time, manufacturing methods were developed that used sulphuric acid as well as nitric acid, making the reaction to nitrocellulose more controllable. The nitrocellulose and camphor were kneaded together like dough, then formed into large blocks to be aged and dried before being processed. Celluloid is a thermoplastic, that is, a plastic which can be reshaped by heat. This gave manufacturers the ability to mold and shape the material in many ways, to stamp it, stretch it, carve it and pierce it. It also could be dyed throughout, unlike many other new materials which could only be coloured on the surface. Filler materials could be used to vary colour, texture, transparency, etc. Celluloid could be machined like wood, heat-molded and blow-molded (this technique is used today for the only commercial product still made of celluloid, table tennis balls).
A great variety of techniques and effects were developed to manufacture celluloid products. For example, because one of the original uses was to make substitutes for ivory and tortoiseshell, ways of creating products looking like these were developed. The celluloid exhibiting ivory-like stripes was made from blocks created using alternating sheets of translucent and more opaque material. These blocks were sliced, pressed together into a new block, and then machined into finished products. A similar process was used to simulate tortoiseshell, starting with cone-shaped pieces of varying colour and transparency. Although celluloid became popular as a substitute material, it soon was appreciated as a new, modern, colourful product.
Despite the great original popularity of celluloid, it is no longer manufactured in quantity. This is primarily due to its flammability, and replacement by safer products. It is also true that celluloid can degrade completely, and this not only destroys the original product but can destroy other materials around it.
The most common type of celluloid degradation is caused by camphor gradually working its way out of the product. Extreme heat will contribute to this process. This causes embrittlement, and in transparent or translucent items deep crazing will be evident. Items affected this way will crumble completely.
More serious degradation occurs when the nitrocellulose degrades, giving off oxides of nitrogen. These combine with water, usually also present when this reaction takes place, forming nitric acid. This is very corrosive to metal, fabric, paper, etc., and often results in substantial destruction of surrounding materials. The effects are worse when the celluloid products are stored in closed containers. The fumes are more concentrated, and the by-products catalyze further reaction, leading to further damage.
People often wonder whether anything can be done to treat celluloid buttons or other products to prevent these types of degradation. Proper storage, in ventilated containers, without excess humidity or high temperatures, will make degradation less likely. Unfortunately, once it has begun it is impossible to reverse. Many celluloid items are stable, but unfortunately it is not possible to know which these will be, although translucent and transparent celluloids are more prone to degradation initiated by ultraviolet light. (Colouring agents which prevent light transmission through celluloid objects appear to give some protection.) Most factors which make degradation likely are present from the time of manufacture, and only proper storage can minimize their effects. Some of these factors include: the purity of ingredient materials; the rinsing and drying processes, which may leave agents catalyzing degradation in the finished materials; the kneading process, which affects the celluloid structure; stability of added materials; and other manufacturing processes.
Celluloid must be stored in ventilated containers, protected from light and humidity. It must be kept separately from other materials, particularly metals, which are attacked by corrosive products of celluloid degradation. Celluloid buttons should be examined periodically and any showing signs of degradation, or affecting surrounding materials, should be discarded. On the bright side, many celluloid buttons have been around for 100 years without showing any signs of degradation, and with proper storage most will stay sound.
Compiled by Sue Dickout, January, 2009
[The primary source for this summary is CELLULOID OBJECTS: THEIR CHEMISTRY AND PRESERVATION by JULIE A. REILLY, Journal of the American Institute for Conservation, JAIC 1991, Volume 30, Number 2, Article 3]